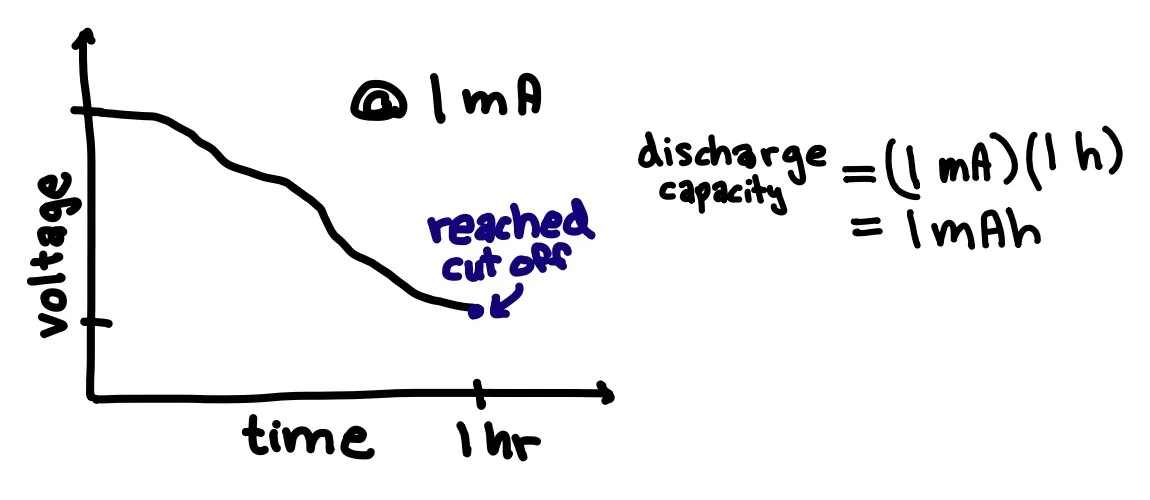
To electrochemically test coin cells, I use instruments to control current and voltage. A common test is constant current cycling in which the battery is charged under constant current to a given high cut-off voltage and then discharged under constant current to a given low cut-off voltage repeatedly. This test tells us the battery’s discharge capacity, which is the discharge current multiplied by the discharge time. If our battery takes one hour to discharge under a discharge current of 1 mA, then the discharge capacity is (1 mA)(1 h) = 1mAh. If a battery can discharge for a longer time at a given current, more energy can be extracted from a battery per discharge cycle. Therefore, we want batteries with high capacities that maintain high capacities for many cycles.

Another test we do a lot is cyclic voltammetry. In cyclic voltammetry, we scan the voltage from a high cut-off value to a low cut-off value and then back up at a specified scan rate. For example, we could scan from 2.4 V to 1.2 V and then back to 2.4 V at a rate of 0.5 mV/s. Due to mass diffusion limitations of reactions occurring at the electrode-electrolyte interface, peaks correspond to electrochemical reactions. Paired with computational techniques, we can assign these peaks to different electrochemical reactions that are expected to occur at that voltage or close by. To find the expected voltage that a reaction occurs at, the free energy change of the reaction must be calculated. Some of the necessary energy values are available from NIST, while others must be calculated using quantum chemistry.