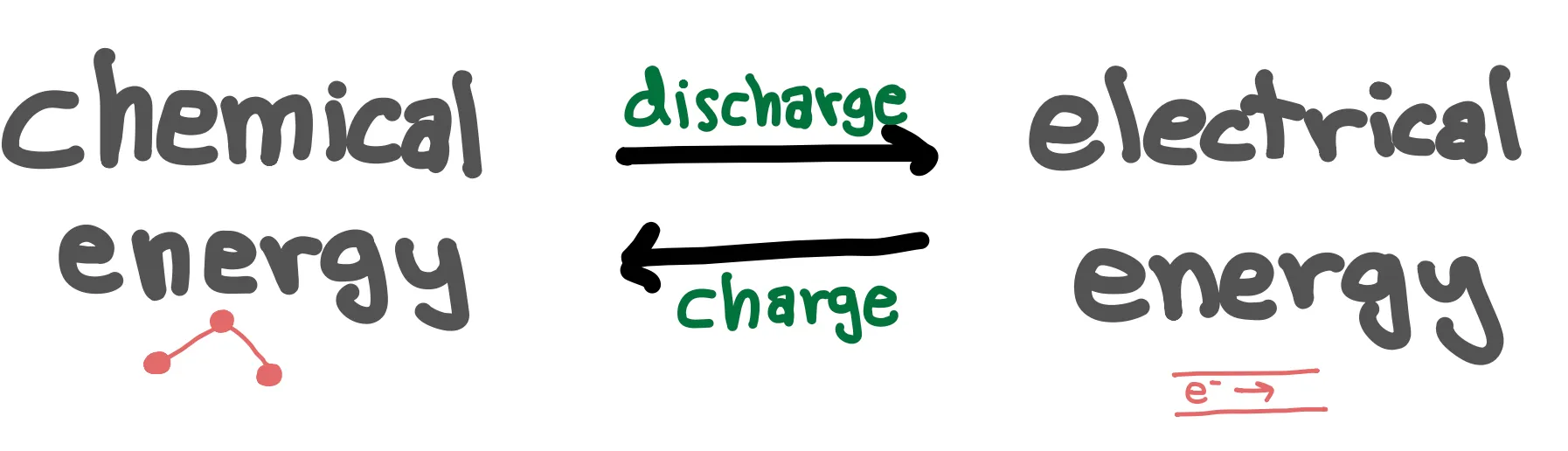
Rechargeable batteries store energy in the form of chemical energy and convert the chemical energy into electrical energy for use by our devices during discharge. In a rechargeable battery, chemical energy is converted into electrical energy during discharge and electrical energy is converted into chemical energy during charge. Electrical energy typically refers to electrons flowing through a wire, while chemical energy is the energy stored in the bonds of atoms and molecules.

A typical battery consists of two electrodes with a potential difference across them. Electrons flow from low to high potential since negative charge is attracted to positive charge. By connecting the electrodes through an external circuit, we thus drive the flow of electrons, or the passing of current. This current then goes on to power your device.
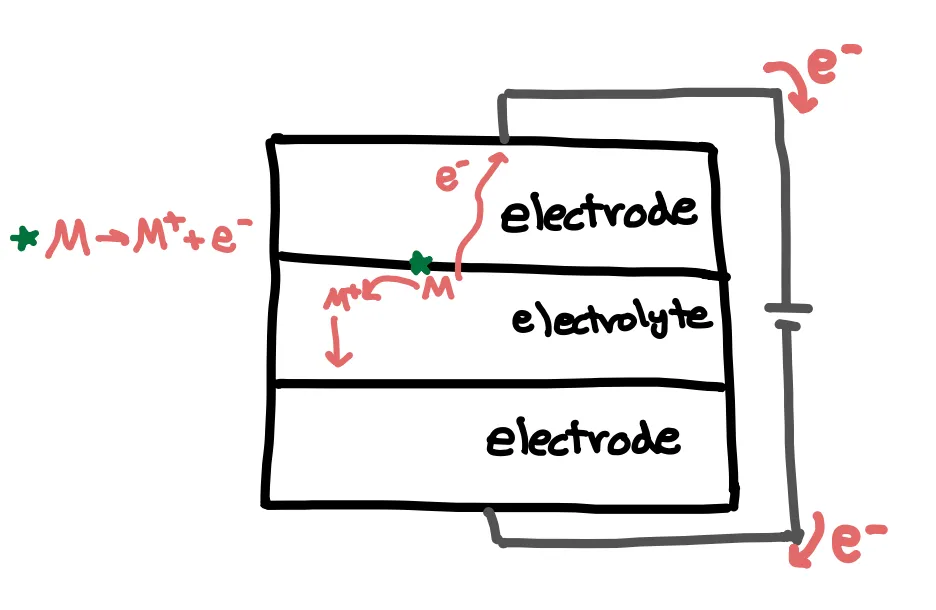
But where do these electrons come from? This is where the chemistry comes into play. Let’s call our element or compound M. At the lower potential electrode, . The electron flows through the external circuit, while the resulting ion migrates through the electrolyte to the higher potential electrode. Here, the ion gains an electron from the external circuit to convert back to ().
That might sound and look a little obscure … let’s look at a real system of a Li-ion battery. Most rechargeable batteries that we use are Li-ion batteries – think phones, computers, and electric vehicles. Since they were first commercialized in 1991, many different Li-ion battery variations have been created. Generally, Li-ion batteries feature carbon anodes and transition metal cathodes. The lithium ions intercalate into the electrodes like books slide into a bookshelf. The processes of charging and discharging can be seen below, with a cobalt oxide cathode. It is key that there are two half cell reactions that are physically separated, one at each electrode, in order to allow the overall reaction to occur while moving electrons through an external circuit.
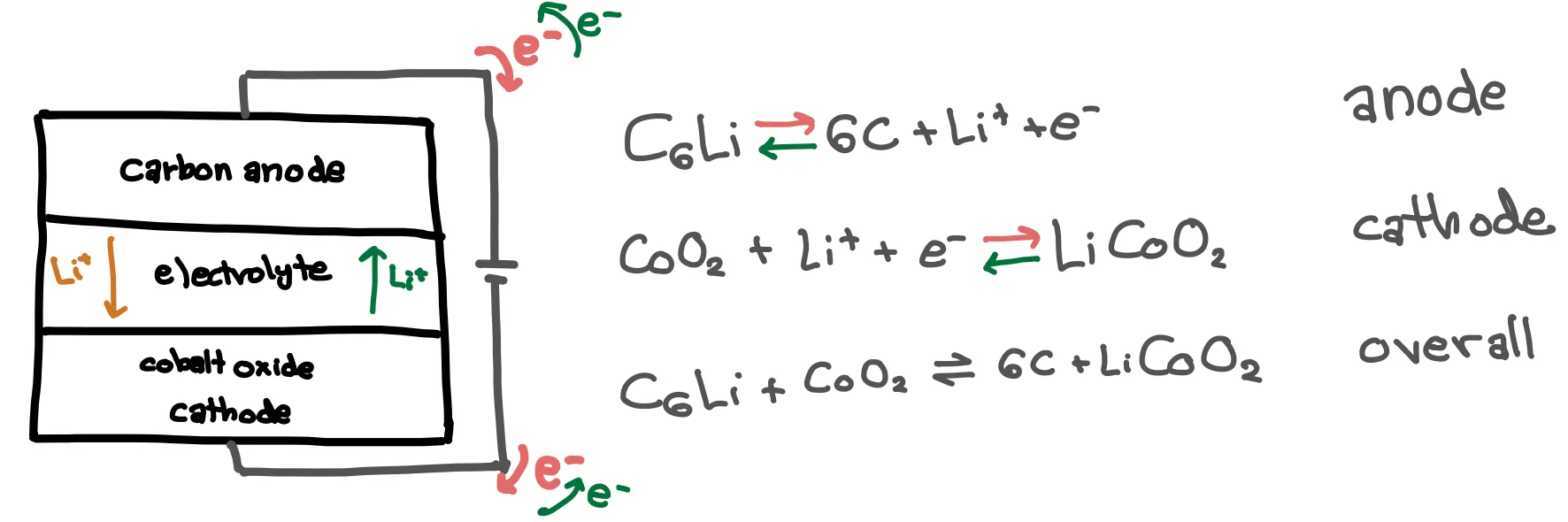
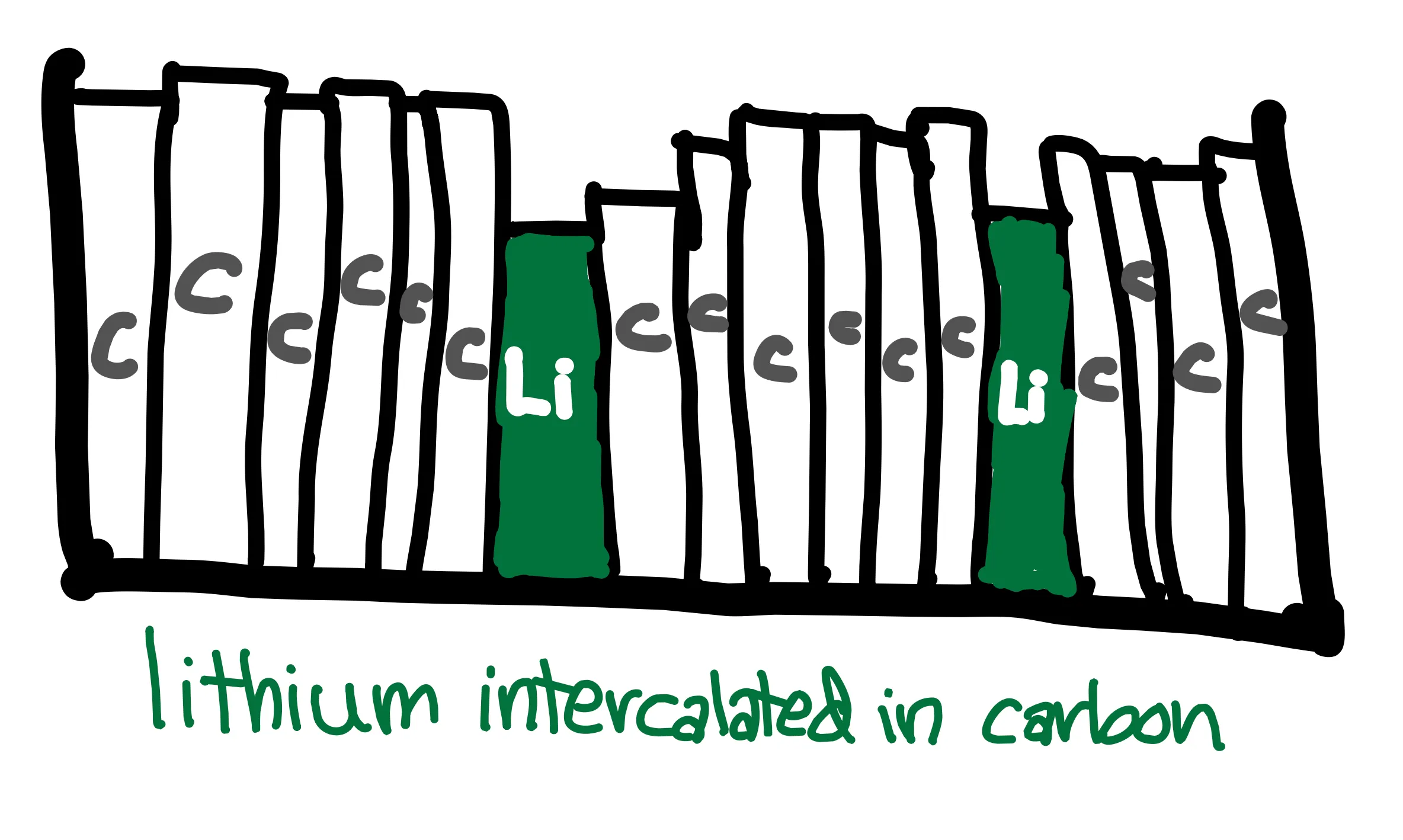
Battery systems differ in the functions that the components have, but generally reactions occur at the electrode-electrolyte interface. The electrode must be able to accommodate ions through intercalation or a conversion reaction (intercalation in a Li-ion battery). The electrolyte allows for the transfer of ions between electrodes, and a separator prevents the electrodes from touching and short circuiting. I discuss the components of a battery in more detail in the next post.