Thermodynamics is the place to start if we want to learn about and understand energy conversion and storage systems!
All you need to know are these two laws.
Let’s talk about the first law to start, using the example of wind power. Wind turbines do not create energy. Rather, they convert the kinetic energy of air molecules to mechanical energy by turning a turbine. The generator then converts this mechanical energy into electrical energy. We can convert one form of energy to another form but not magically generate energy.
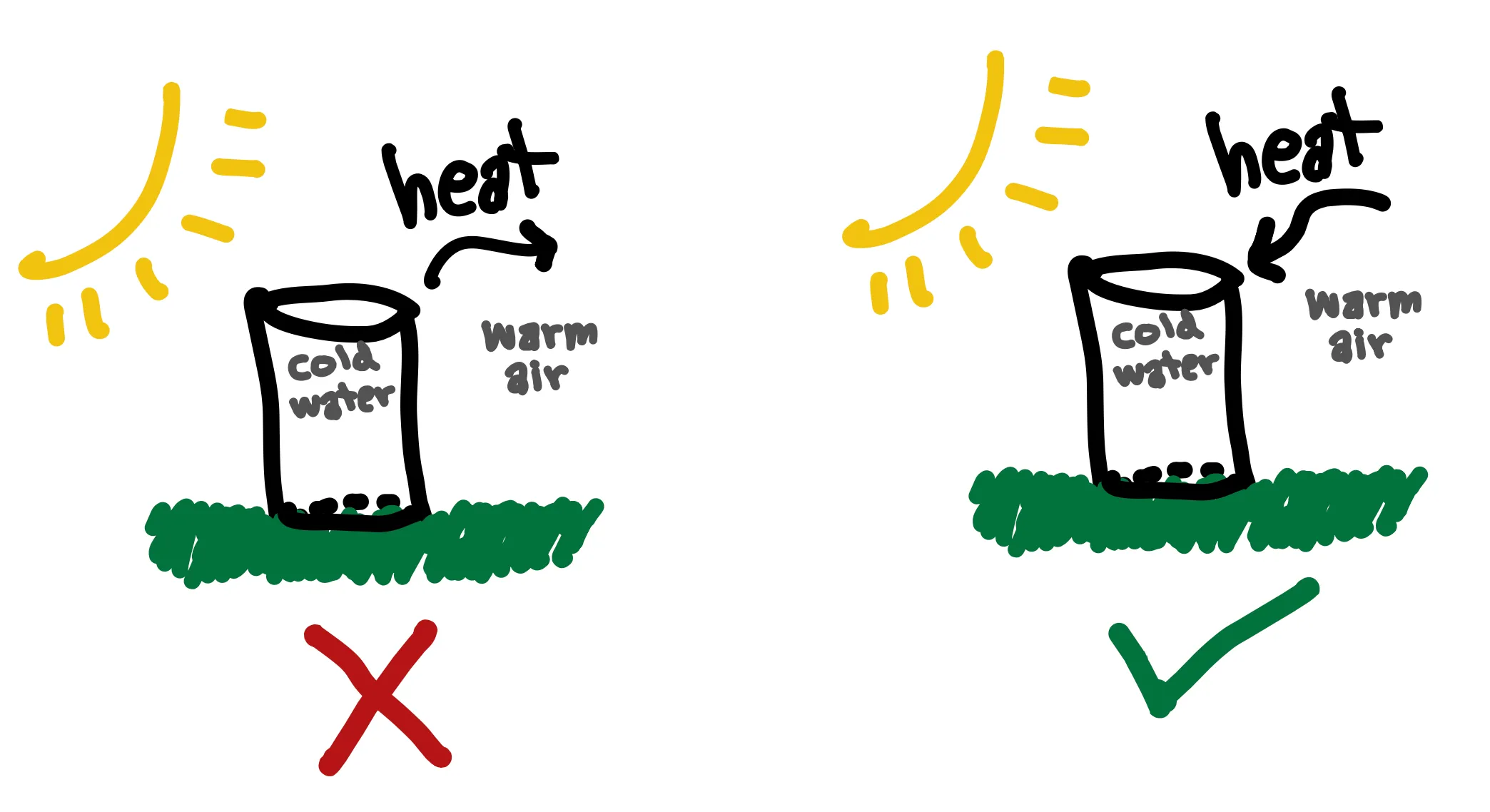
On to the 2nd law. There are many forms of the 2nd law (which can all be shown to be equivalent), so I chose the one that I think is most intuitive to understand with no prior thermo experience, which is Clausius’s statement. Let’s say you fill up a glass of iced water and take it outside on a warm day. Heat transfers from the surrounding air to the water to melt the ice and increase the water temperature until the water reaches the temperature of the ambient air. Heat spontaneously transfers from the warmer body, the air, to the colder body, the iced water. If heat were to flow from the colder body to the warmer body in this situation (violating the 2nd law), the iced water would be spontaneously getting colder, which we know doesn’t happen. This simple statement governs the direction of the spontaneous flow of energy and limits what is possible in energy systems.

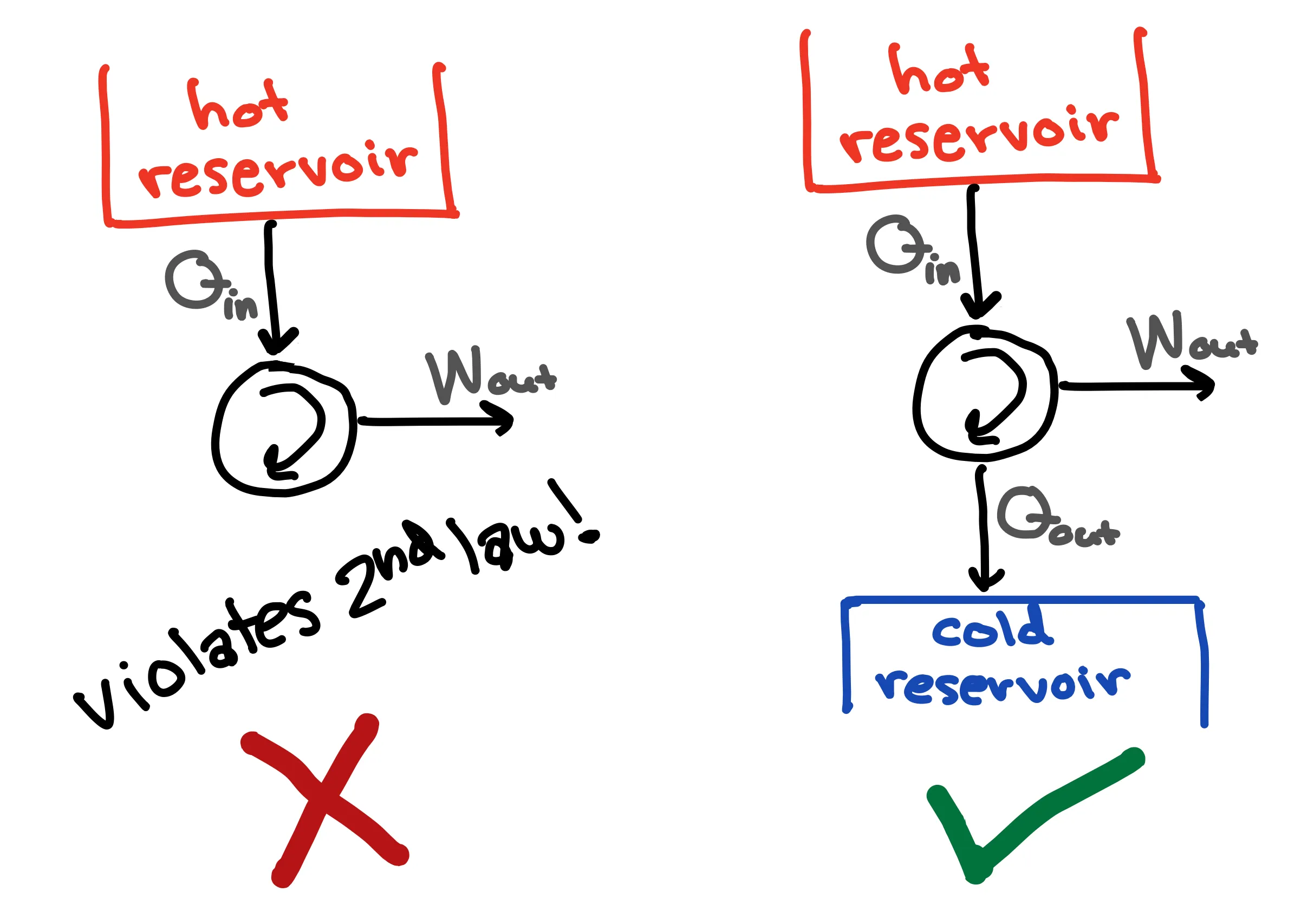
Clausius’s statement can be shown to be equivalent to the Kelvin statement, which states that it is impossible to convert heat completely to work. If we transfer one unit of energy via heat from a high-temperature reservoir, we can’t convert the full unit of energy to electric energy. Rather, some of the energy must be released via heat to a low-temperature reservoir (such as the surrounding air). This is super important when considering heat engines and pumps and many other energy systems.
I know I glossed over some terminology and reasoning in this short introduction of thermodynamics, but hopefully this gives you an idea of the general limitations imposed by nature on how we can use energy. To begin to evaluate energy conversion and storage systems, we must first think about their feasibility and efficiency using thermodynamics. If you want to learn more, I recommend the thought-provoking book Four Laws that Drive the Universe by Peter Atkins. Atkins explores the history of and implications of the laws in more depth, introduces more terms used in thermodynamics, and digs into what these terms and laws really mean. What really is temperature? Can you define energy?